X-ray physics notes curriculum
Fundamentals of radiation (current module)
The X-ray machine
Production of X-rays
Interaction of radiation with matter
X-ray detection and image formation
Image quality
Radiation safety in X-ray imaging
Fluoroscopy
Mammography
One of the most important concepts in physics is that electromagnetic radiation exhibits both wave-like and particle-like behaviour.
This is called wave–particle duality.
It may seem abstract at first, but it explains many of the key behaviours of X-rays. For example, we say that photons collide with electrons. This implies the photon is a particle.
However, when we talk about scattering of X-rays it makes much more sense to think of these as being waves.
So let’s look at the different models.
The wave model (less relevant in diagnostic X-ray imaging)
The wave model describes electromagnetic radiation as a continuous oscillation of sinusoidal electric and magnetic fields characterised by:
- Wavelength (λ): the distance between successive wave peaks.
- Frequency (f): the number of oscillations per second.
- Amplitude: related to intensity.
Wave behaviour includes:
- Reflection – change in direction at an interface.
- Refraction – change in velocity when passing between media.
- Diffraction – bending around obstacles.
- Interference – constructive or destructive overlap of waves.
These behaviours are negligible in routine imaging because X-ray wavelengths (~0.05 nm) are extremely small compared with anatomic structures. X-ray diffraction, however, does form the basis of X-ray crystallography).
The particle model (photon model)
The particle model treats electromagnetic radiation as a stream of discrete quanta (packets of energy), or photons, each carrying energy:
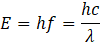
Key features:
- Photons have no rest mass or charge.
- Each photon interacts independently with matter.
- Interactions: Photons can collide with electrons and eject them from atoms (Compton scatter and the photoelectric effect), or pass through without interaction.
- Energy transfer occurs in discrete amounts, not continuously.
Quantum physics
The duality reflects the quantum nature of electromagnetic radiation.
Photons behave as waves when propagating, but their energy is transferred in discrete quanta during interactions. This explains why energy deposition in tissue is probabilistic rather than continuous.
There’s no need to get too caught up in these concepts. Just know that wave-particle duality exists and why it is relevant for diagnostic X-ray imaging.
Key takeaways:
- Dual behaviour: EM radiation behaves like both a wave and a particle.
- Wave model → explains how radiation travels.
- Particle model → explains how radiation interacts with matter.
- In diagnostic X-ray physics, the photon (particle) model dominates.
Exam tips:
There’s no too much that can trick you here. A common type of question centres around which model is responsible for the photoelectric effect. As we’ve said it’s the particle model. This probably comes up often because the first experiment showing that X-rays act as a particle used the photoelectric effect to make the conclusion.
I will say keep this concept in mind later on. Examiners may use these concepts as distractors. For example, in shielding of X-rays (like using lead aprons) the effect is binary. Either the photon is absorbed or transmitted. There is no partial absorption of an individual photon.
So where to next?
A key concept in X-ray imaging is how electromagnetic waves (i.e. X-rays) are created and how they interact with matter. These interactions occur at the atomic level. It’s important to understand the basic structure of the atom to have a good grasp of x-ray production and interaction with matter. That’s what we’re going to look at next, basic atomic structure.
