X-ray physics notes curriculum
Fundamentals of radiation (current module)
The X-ray machine
Production of X-rays
Interaction of radiation with matter
X-ray detection and image formation
Image quality
Radiation safety in X-ray imaging
Fluoroscopy
Mammography
Electromagnetic (EM) radiation is energy transmitted through space as oscillating electric and magnetic fields, oriented at right angles to each other and to the direction of propagation. All forms of EM radiation travel at the speed of light in a vacuum (c = 3 x 108 m s-1).
The Electromagnetic Spectrum
The electromagnetic spectrum encompasses a continuous range of radiation types, differing only in wavelength (λ), frequency (f), and photon energy (E). These quantities are related by:
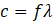
- c: speed of light (3×108 m/s)
- f: frequency (Hz)
- λ: wavelength (m)
In a given medium, speed is constant. Thus, higher frequency = shorter wavelength.
The main regions of the spectrum are:
| Region | Typical wavelength range | Examples / Applications |
|---|---|---|
| Radio waves | > 1 m | Broadcasting, MRI radiofrequency pulses |
| Microwaves | 1 m – 1 mm | Communication, heating, radar |
| Infrared (IR) | 1 mm – 700 nm | Remote controls, thermal imaging |
| Visible light | 700 – 400 nm | Optical imaging, illumination |
| Ultraviolet (UV) | 400 – 10 nm | Sterilisation, fluorescence |
| X-rays | 0.1 – 0.01 nm | Diagnostic and therapeutic imaging |
| Gamma rays | < 0.01 nm | Nuclear medicine, radiotherapy |
Diagnostic X-rays correspond to wavelengths on the order of 0.01–0.1 nm, with photon energies in the range of 20–150 keV. As we’ve seen wavelength and frequency are dependent factors. Photon energy is directly related to frequency. As a result, electromagnetic radiation with shorter wavelengths (and by definition higher frequency) have higher energy. Let’s look at the relationship between energy and frequency.
Photon Energy
Photon energy is quantised and determined by Planck’s relation:
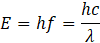
where:
- h = Planck’s constant (6.63×10-34 Js)
- f = frequency
- λ = wavelength
This simple equation underpins much of X-ray physics. Increasing the frequency (or decreasing the wavelength) means higher photon energy.
For the astute amongst you, you’ll notice that energy values are discrete because of Planck’s’ constant. All photon energies are a multiple of Planck’s’ constant. This is why we describe photons as packets of energy. Without getting too into the weeds, this discrete nature of photons is representing the quantum nature of electromagnetic radiation. As you’ll see next, electromagnetic waves can be thought of as both continuous waves and discrete particles. This is one of many defining key properties of EM radiation. Let’s review these properties.
Key properties of electromagnetic radiation:
- No mass, no charge
- Unlike electrons or protons, photons have no rest mass or charge. This allows them to travel freely through space but also makes their interactions probabilistic rather than deterministic.
- Speed of light
- In a vacuum, all EM radiation travels at 3×108 m/s3.
- In matter, speed is reduced depending on refractive index.
- Oscillating fields
- EM radiation consists of perpendicular electric and magnetic fields oscillating at the same frequency, propagating energy forward.
- Penetration depends on energy
- Low-energy photons (e.g. visible light) are easily absorbed by tissue. Thats why we create shadows and light doesn’t travel right through us.
- High-energy photons (X-rays) have enough energy to penetrate and interact at a deep tissue level, this enables X-ray imaging.
Key takeaways:
- Electromagnetic radiation consists of photons, each carrying energy defined by Planck’s relation.
- Diagnostic X-rays occupy the high-energy end of the EM spectrum.
- Energy, frequency, and wavelength are tightly linked; changing one changes the others.
- Photon energy determines how radiation interacts with tissue and whether it is useful for imaging.
Exam tips:
Exam questions often centre around understanding the relationship between frequency, wavelength and energy.
Here are some common points of confusion.
- Higher frequency = shorter wavelength
- Frequency of an electromagnetic wave is constant once it is created, the wavelength and speed of the wave change (very slightly) as it propagates through different tissues. This is far more pronounced and clinically relevant in ultrasound imaging.
- Higher frequency = higher photon energy = more penetrating photon.
- The higher the photon energy the less likely it is to interact with tissue. (We’ll discuss this relationship in great depth when we discuss the photoelectric effect).
- Typical diagnostic X-ray energy is 20-150 keV (remember the units).
- Typical wavelength in diagnostic x-rays is in the range of 0.01 – 0.1 nm (it’s useful to remember x-rays have sub-nanometer wavelength).
Whats next?
Ok, moving swiftly on. I mentioned here that X-ray photons can be thought of as both waves and particles. Let’s take a closer look at this concept next!
