X-ray physics notes curriculum
Fundamentals of radiation (current module)
The X-ray machine
Production of X-rays
Interaction of radiation with matter
X-ray detection and image formation
Image quality
Radiation safety in X-ray imaging
Fluoroscopy
Mammography
Atoms are the fundamental units of matter. X-ray imaging is fundamentally about how photons interact with atoms in matter, especially electrons in their shells. The properties of atoms (their composition, electron arrangement, and binding energies) dictate how, and in what proportion, X-rays are absorbed, scattered, or transmitted.
The atom
- Nucleus
- Made of protons (positive charge) and neutrons (no charge). These particles are known as nucleons.
- Nearly all the mass of the atom is concentrated here.
- The number of protons = atomic number (Z) → defines the element. For example, any atom with a total of 6 protons is called “Carbon” regardless of the number of neutrons or electrons. This comes up often in exams, so I’ll say it again, it’s the number of protons (the atomic number) that defines the atomic element.
- Electrons
- Negatively charged, extremely light particles surrounding the nucleus.
- Arranged in shells (K, L, M …) and within those shells, orbitals.
Electron shells and orbitals
- Shells: Grouped energy levels labelled K (n=1), L (n=2), M (n=3) … n denotes the principal quantum number.
- Orbitals: Subdivisions within shells where electrons are most likely found. These are not exact paths that the electrons take, rather regions where electrons are most likely to be at any given point in time.
- s orbitals: spherical
- p orbitals: dumbbell-shaped
- d, f orbitals: more complex shapes
Electron filling rules:
- Aufbau principle: Electrons fill orbitals starting from the lowest energy level (1s → 2s → 2p → 3s …).
- Hund’s rule: Within a subshell (e.g. p orbitals), electrons occupy orbitals singly before pairing, to minimise electron repulsion.
Clinical tie-in: Although orbital detail matters less directly in diagnostic radiology, these rules explain why elements have characteristic binding energies and subsequently create very specific characteristic x-rays (we’ll discuss this later on in the X-ray production lessons).
Binding energy
This is a key concept.
- Electrons are held in orbitals by the positive charge of the nucleus.
- Binding energy = energy required to remove an electron from its orbital.
- Increases with:
- Closer shells (K > L > M)
- Higher atomic number (Z).
- Key for X-ray interactions: a photon must exceed the binding energy to eject an electron (photoelectric effect, characteristic radiation).
Defining elements
The following terms can cause confusion in exams. Examiners know this and use this to test true understanding of terms. Let’s get clear about each of the following. These are easy marks!
- Isotope: Same number of protons (same Z), different number of neutrons.
- Example: C-12 and C-14.
- Isotone: Same number of neutrons, different number of protons.
- Example: Carbon-14 and Nitrogen-15 (both 8 neutrons).
- Isobar: Same total nucleon/mass number (A), different Z and N.
- Example: C-14 and N-14 (both A = 14).
- Nuclide: A general term for a nucleus specified by both Z and A.
Nuclide notation
Elements can be described in a standardised way. It’s useful to be comfortable reading this notation (especially if you’re also studying nuclear medicine physics later on.)
Nuclide notation is as follows:
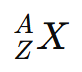
Where:
- X = chemical symbol
- Z = atomic number (protons)
- A = mass number (protons + neutrons)
Example:
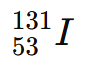
= Iodine-131 → 53 protons, 78 neutrons, used in nuclear medicine.
Why does all this matter in X-ray Physics?
- X-ray production: Characteristic X-rays occur when orbital vacancies are filled; the photon energy equals the difference in binding energies between shells..
- X-ray interactions: Photoelectric absorption depends on orbital binding energies, especially in high-Z materials like bone or contrast agents.
- Clinical practice: Iodine and barium are chosen as contrast media because their inner-shell binding energies fall within the diagnostic X-ray range.
- Target design: tungsten (Z = 74) provides efficient X-ray production and high thermal resistance.
Key takeaways and exam tips
- Atomic number (Z) = number of protons → defines the element. Don’t confuse this with mass number (A) = protons + neutrons.
- Binding energy increases with Z and is higher for inner shells (K > L > M).
- Isotope vs isotone vs isobar:
- Isotope: same Z, different neutrons.
- Isotone: same neutrons, different Z.
- Isobar: same A, different Z.
- Nuclide: a general term for a defined nucleus.
- Nuclide notation: Don’t mix up the subscript and superscript → subscript = Z, superscript = A.
- Orbitals: Know that electrons occupy subshells (s, p, d, f) following the Aufbau principle (lowest energy first) and Hund’s rule (fill singly before pairing). You don’t need to know quantum mechanics detail, just the rules.
- Clinical tie-ins:
- Iodine and barium are used as contrast because their binding energies fall within diagnostic X-ray energies.
- Tungsten target chosen for its high Z and high melting point.
- Likely pitfalls:
- Confusing atomic number vs mass number.
- Forgetting that isotopes are the same element (same Z).
- Mixing up which shell has the higher binding energy (K > L > M).
Up next?
Next, we’ll define and compare the quantities and units used to describe radiation exposure and dose.
